Abstract
Allogeneic hematopoietic stem cell (HSC) transplantation is one of the curative therapeutic options for patients with a variety of inherited disorders of blood cells, including immune deficiencies and hemoglobinopathies. In addition, considerable progress has been made toward achieving clinical benefit from autologous HSC ex vivo gene therapy for some of these disorders. Generally, some level of conditioning using chemotherapy and/or radiation is needed to achieve the required engraftment of allogeneic HSC or gene corrected autologous HSC. There is considerable interest in finding less toxic and more focused approaches to achieve the conditioning. Promising results were observed using antibody-based approaches including anti-c-kit (CD117) or anti-CD45 which directly target HSCs, and these results can be enhanced by linking the antibody to a toxin such as saponin. Here, we explored a related approach in mouse models in which chimeric antigen receptor (CAR)-T cells targeting c-kit (c-kit CAR-T cells) were employed as conditioning to achieve significant engraftment of congenic HSCs. Our initial attempts at efficient conditioning with c-kit CAR-T cells failed because there was insufficient trafficking of CAR-T cells to bone marrow (BM) and insufficient CAR-T cell expansion in vivo and because we did not eliminate the CAR-T cells before transplanting congenic HSCs.
To address these barriers, before injecting CAR-T cells (derived from C57BL/6 Thy1.1/CD45.2 mouse spleen on Day1 and transduced with CAR retrovirus on Day2) we pre-treated the recipient mice (Thy1.2/CD45.2) with low-dose cyclophosphamide (CY, 125 mg/kg; Day2) and co-transduced the c-kit CAR-T cells with a CXCR4 (CD184) expression vector (Day2), followed by injection of 5×106 CAR-T cells on Day3. We later removed these CAR-T cells by treating the mice with anti-Thy1.1 antibody (Ab) on Day11 prior to HSC transplantation from congenic Thy1.2/CD45.1 mice (Day12; 3×106 cells/mouse).
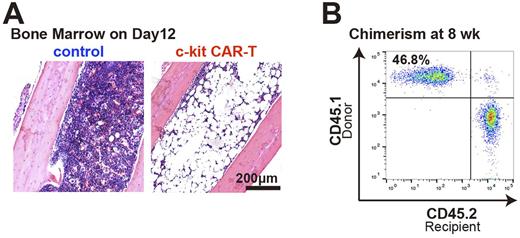
After single CAR retrovirus transduction, >90% of T cells expressed CAR. When used in vitro, CAR-T cells efficiently depleted BM c-kit+ population (10.5% reduced to 1.6%) and completely suppressed colony formation in nitrocellulose medium. In initial in vivo studies, we used a luciferase marker in the CAR-T cells to allow whole body imaging in addition to flow cytometry (FACS) of BM to assess trafficking. With no pre-treatment of mice, both in vivo imaging and FACS demonstrated poor trafficking of CAR-T cells to BM (< 1% of cells), and there was no decrease of the BM c-kit+ population. In contrast, pre-treatment with low-dose CY and overexpression of CXCR4 on the CAR-T cell surface improved the CAR-T cell expansion in vivo and the migration into BM (mean Thy1.1+ cells, 11.9%; n= 5), and this was associated with significant in vivo depletion of BM c-kit+ population (9.0% reduced to 0.0%). Severe pancytopenia was confirmed in peripheral blood (Hgb 2.2 g/dL compared to the normal, 13.0 g/dL) along with aplastic BM (Figure A), and these mice died within 4 weeks without BM transplant. Subsequent injection of anti-Thy1.1 Ab completely depleted CAR-T cells in viv o. When congenic BM cells were transplanted into mice conditioned in this manner (CY+CAR-T+Thy1.1 Ab), these mice achieved complete recovery of hematopoiesis with significant engraftment of donor BM in all lineages in peripheral blood (Figure B) and long-term HSCs in BM (> 20 % donor chimerism).
Our findings provide proof-of-concept that c-kit CAR-T cells can be used to achieve sufficient conditioning to allow donor chimerism after congenic transplantation without use of high-dose chemotherapy or radiation. Low-dose CY was required to achieve the reduction of host T cells and stimulate in vivo expansion of CAR-T cells. A key element to achieve BM HSC depletion was the co-transduction of CXCR4 that greatly increased trafficking of the CAR-T cells to BM. This finding may be one of the most important aspects of this study, with broader implications for the CAR-T cell field in suggesting that use of trafficking receptors or ligands might be generalizable as a method to enhance targeting to designated organs or solid tumors. In summary, our studies add to the list of tools that should be further explored in the effort to seek less toxic conditioning regimens for BM transplantation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.